In the past, cancer was considered a single disease characterized by the uncontrolled growth of cells. However, the advancement in genetics and proteomics in recent times has revealed that cancer is a combination of various diseases. Nevertheless, cancers share several common features which are wonderfully described by Hanahan and Weinberg in their review “Hallmarks of Cancer”. For a long time, cancer researchers were focused on finding a single magic bullet for cancer treatment. The notion has now become obsolete and the contemporary cancer therapy consists of combinations of two or more drugs to target multiple hallmarks of cancer. In a recent paper published in the journal Nature Communications, Haikala et al. used a
So, what is MYC-oncogene?
MYC is a transcription factor downstream of various growth stimuli and regulates several features of cancer including proliferation, metabolism, apoptosis, immune evasion etc. It is overexpressed in almost half of the human cancers and its overexpression is liked to poor clinical outcome. A drug targeting MYC has long been sought by researchers but has still remained an uphill battle because of its structural complexity. This has led researchers to come up with alternative strategies to target MYC in cancers, such as targeting factors downstream of MYC.
MYC and BCL-2 family proteins in breast cancer

To look for targetable factors downstream of MYC, Haikala et al. analyzed the protein level of Myc and BCL-2 family members using tissue microarray. To refresh your memory of BCL-2 family proteins, read my previous post on Apoptosis inducers. The group found that half of the samples expressed high levels of Myc, of which, more than half also expressed higher levels of BCL-2 family proteins. Encouraging the testing of BCL-2 inhibitors in Myc high breast cancer.
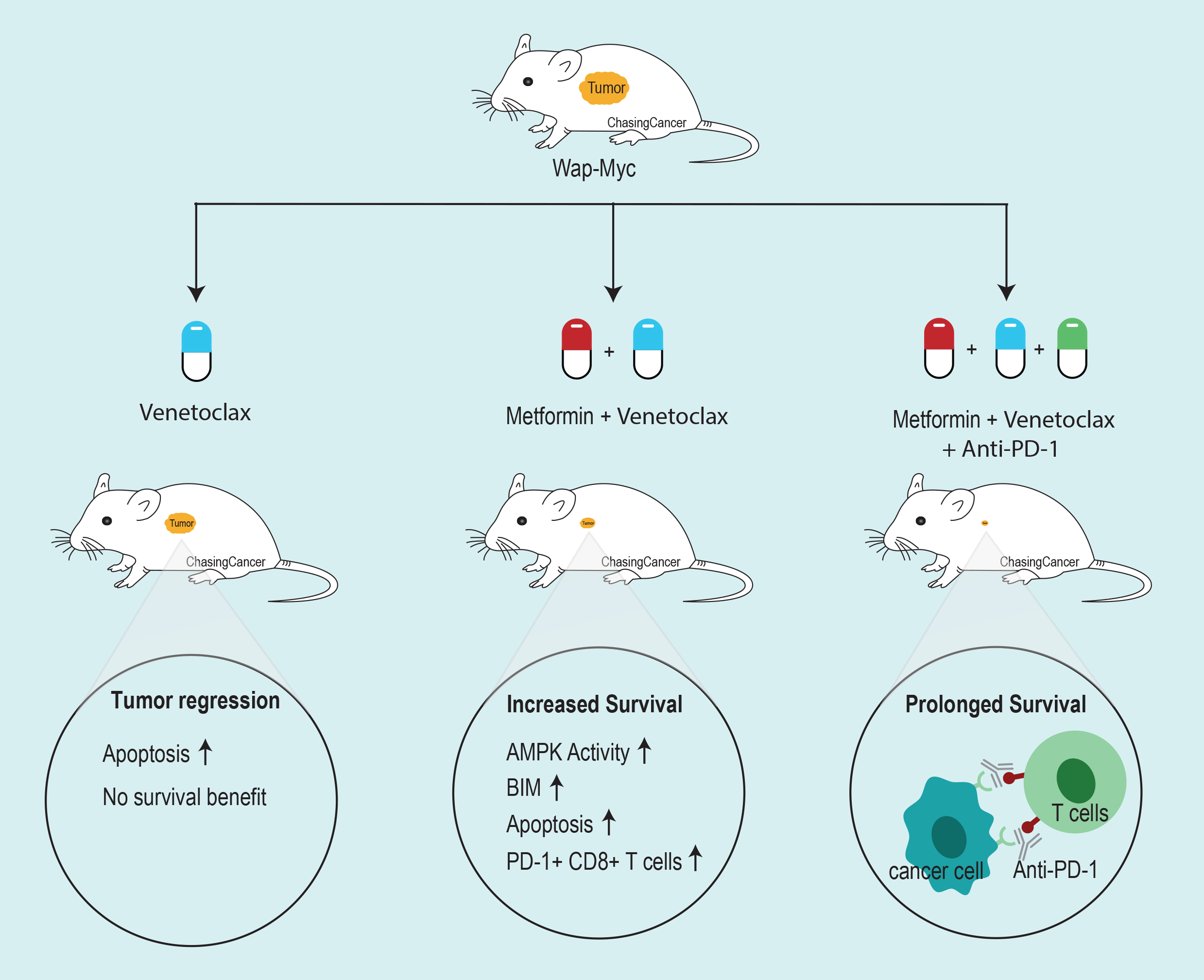
BH3 mimetic ABT-737 inhibit the tumor growth but didn’t increase survival in Myc-driven mouse model of breast cancer
To access the efficacy of BH3 mimetics in Myc-driven tumors, the group used autochthonous and orthotopically
Boosting the apoptotic potential of ABT737

Owing to the modest effect of ABT-737 in vivo, Haikala et al. did a screen to boost the apoptotic potential of ABT-737. They found that anti-diabetic drug Metformin-induced Myc-dependent apoptosis when combined with ABT-737 in MCF10A cells. They further confirmed that metformin was able to activate AMPK, a known target of metformin. AMPK activation leads to upregulation of pro-apoptotic protein BIM, sensitizing MYC-driven tumors to BH3 mimetics.
Metformin and Navitoclax combo in vitro
Navitoclax is an orally available analog of ABT-737. Haikala et al. tested the apoptotic potential of metformin and Navitoclax in a panel of 16 triple negative breast cancer cell lines. They found a positive correlation of Myc expression with the apoptotic potential of the combo.
Combo in vivo
To test the potential of Navitoclax and metformin combination in vivo, Haikala et al. used patient-derived xenograft models derived from metastatic TNBC patient samples. The combination was able to inhibit tumor growth in vivo, conferring a survival benefit to mice transplanted with Myc high TNBC specimens. They also treated immunocompetent
Immune evasion
Although the combination treatment restricted growth and prolonged survival, the mice succumbed to death post-treatment. After the careful examination of the post-treatment tumor samples, Haikala et al. found leukocytes enrichment in the treated tumors including CD4+ T cells, NKT cells, and NK cells. However, active cytotoxic T cells (CD8+/CD107+) were low in treated tumors compared to control. Contrary to this, interferon-gamma secreting T cells were high in treated tumors. Since interferon-gamma also promotes T cell exhaustion by upregulating PD-1/PD-L1, the group analyzed the cytotoxic T cells and found increased in PD-1 positive T cells. Suggesting, T-cell exhaustion in combination treated samples.
Introduction of checkpoint inhibitor to the combo

Since T-cell exhaustion could be the reason for post-treatment tumor recurrence, Haikala et al. introduced anti-PD-1 inhibitor to the metformin and Venetoclax (BCL-2 specific inhibitor with fewer side effects) combination. To access the efficacy of anti-PD-1 therapy, the group treated the syngrafted mice with neoadjuvant Metformin and Venetoclax combination followed by surgical removal of the tumor and treatment with adjuvant therapy combining the existing combo with anti-PD-1 therapy. Anti-PD-1 therapyy didn’t give any significant survival benefit alone as adjuvant therapy. However, in combination with Metformin and Venetoclax, anti-PD-1 therapy demonstrated a dramatic response with all mice in the group surviving the 30 days follow-up period. The group then extended the follow-up period to 60 days and compared their Venetoclax and Metformin combo + anti-PD-1 therapy with Paclitaxel + anti-PD-1 therapy. They found that Venetoclax and metformin combination decreased tumor volumes compared to Paclitaxel + anti-PD-1 therapy and induced less liver injury markers compared to Paclitaxel.
To summarize, Haikala et al. demonstrate that Myc high tumors can be sensitized to apoptotic inducers like BH3 mimetics by imposing AMPK-mediated BIM upregulation. The combination was able to increase the survival in mice, however, tumors came back post-treatment. One of the reasons could be T-cell exhaustion indicated by increased in interferon-gamma positive T cells with increased PD-1 expression. Combining anti-PD-1 therapy with Metformin and Venetoclax combo prolonged survival. Suggesting, apoptosis mediated immune activation as a possible therapeutic strategy to target Myc-dependent tumors.
By Shishir Pant
Reference
Haikala, H. M., et al. (2019). “Pharmacological reactivation of MYC-dependent apoptosis induces susceptibility to anti-PD-1 immunotherapy.” Nat Commun 10(1): 620.